Applications of imidazole and its derivatives
Introduction
Imidazole is a five-membered aromatic heterocyclic compound with two nitrogen atoms. The hydrogen atom moves between the two nitrogen atoms, so there are two reciprocal isomers. Imidazole is not only present as histidine in living organisms, but is also a constituent of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) purines. The imidazole molecule itself is characterized by good electron transfer and easy functionalization. Many compounds containing the imidazole ring have good biological activity and play an important role in the field of medicine and pesticides.
Many drugs also contain imidazole ring, such as nitroimidazole and imidazole antifungal agents. In recent years, imidazole building blocks have become a research hotspot for the synthesis and application of new nitrogen-containing heterocyclic compounds at home and abroad. The imidazole ring structure is widely present in biological molecules, such as histidine and the corresponding Holmont histamine. 1-5
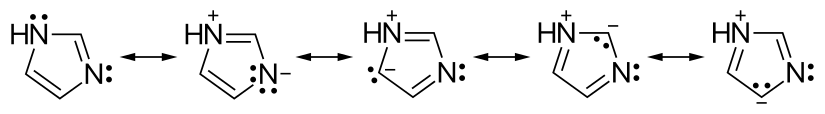
Fig 1. Resonance structure of imidazole
Pharmaceutical Chemistry Applications
There are a wide variety of biomolecules that contain imidazole functional groups. The most common is the amino acid histidine, which contains an imidazole side chain. Histidine is one of the 20 most prevalent amino acids in living organisms, and as such is present in many proteins and enzymes, and is also an important component that influences the liganding ability of hemoglobin.
Imidazole-based histidine plays a very important role in intracellular buffering.6 Histidine can be decarboxylated to form histamine, a common biomolecule with functions such as lowering blood pressure and contracting the uterus. Histamine released by cells causes hives when produced in allergic reactions.
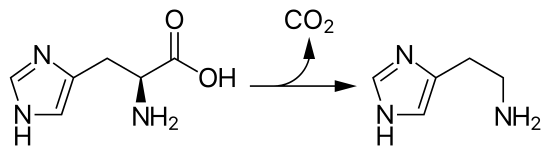
Fig 2. Histidine to Histamine Reaction
At the same time, it has been found that some drugs can selectively act on the imidazoline receptor and reduce the effect on the α2 receptor, thus avoiding the side effects caused by colistin, such as Moxonidine.
Pharmaceutical Derivatives
Substituted imidazole derivatives are of great value in the treatment of many systemic fungal infections.7 Imidazoles such as ketoconazole, miconazole and clotrimazole belong to the azole class of antifungal drugs.
In contrast, fluconazole, itraconazole and voriconazole belong to the triazole group. The differences between imidazoles and triazoles also lead to differences in the mechanism of cytochrome P450 enzyme inhibition. The N3 of imidazole compounds binds to the heme iron atom in the ferric cytochrome P450, whereas the N4 of triazoles binds to its heme group. For cytochrome P450, triazoles are more biospecific than imidazoles, making them more effective than imidazoles.8
Some imidazole derivatives are useful against insects, e.g., sulconazole nitrate is highly antifeedant against Australian carpet beetle larvae Anthrenocerus australis, which can digest keratin, and econazole nitrate is effective against the common clothes moth Tineola bisselliella.9
Industrial Applications
Imidazole itself has few direct applications. Instead, it is a precursor to a variety of agrochemicals, including enilconazole, climbazole, clotrimazole, prochloraz, and bifonazole. 10
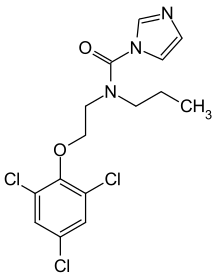
Fig 3. Imidazole agrochemicals - Prochloraz
Coordination Chemistry Applications
An important application of imidazole in biochemistry, on the other hand, is the purification of His-tag proteins in metal chelate affinity chromatography (IMAC). The His-tagged protein binds to the nickel ion medium in the bead pore on the surface of the column, and the excess imidazole passes through the column, displacing the His-tag protein from the nickel ligand, and ultimately purifying to obtain the target protein with high purity. The (PBIS) derivatives of 4H-pyrrolo[1,2-a]benzimidazole are a class of nitrogen heterocyclic compounds. It has been shown that the cyano (-CN) and ethyl (-CO2Et) substituents on the pyrrole ring of PBIS have good fluorescence-assisted coloration, whereas the phenoxycarbonyl (-COOPh) and benzyl (-COOMe) groups are quenching.
Reference
1. Katritzky; Rees. Comprehensive Heterocyclic Chemistry. Vol. 5, p.469-498, (1984).
2. Grimmett, M. Ross. Imidazole and Benzimidazole Synthesis. Academic Press, (1997).
3. Brown, E. G. Ring Nitrogen and Key Biomolecules. Kluwer Academic Press, (1998).
4. Pozharskii, A. F., et al. Heterocycles in Life and Society. John Wiley & Sons, (1997).
5. T. L. Gilchrist. Heterocyclic Chemistry. The Bath press. 1985. ISBN 978-0-582-01421-3
6. Hochachka, P. W.; Somero, G. N. (2002). Biochemical Adaptation: Mechanisms and Process in Physiological Evolution. New York: Oxford University Press.
7. Leon Shargel (2007). Comprehensive Pharmacy Review (6th ed.). p. 930. ISBN 9780781765619.
8. Davis, Jennifer L.; Papich, Mark G.; Heit, Mark C. (2009). "Chapter 39: Antifungal and Antiviral Drugs". In Riviere, Jim E.; Papich, Mark G. (eds.). Veterinary Pharmacology and Therapeutics (9th ed.). Wiley-Blackwell. pp. 1019–1020. ISBN 978-0-8138-2061-3.
9. Sunderland, M. R.; Cruickshank, R. H.; Leighs, S. J. (2014). "The efficacy of antifungal azole and antiprotozoal compounds in protection of wool from keratin-digesting insect larvae". Textile Res. J. 84 (9): 924–931. https://doi.org/10.1177/0040517513515312
10. Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. (2002). "Imidazole and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. https://doi.org/10.1002/14356007.a13_661.
